Blog #4 – Ocean Acidification
There have been many serious assaults on the shellfish populations of coastal areas since the
coming of Europeans to the North American continent in the early 17th century. For example, the
European green crab, introduced in the 19th century, has virtually eliminated the soft shell clam
population in some areas and continues to threaten New England populations. Oysters were
over-harvested and oyster reefs were destroyed by dredging to the point where the oyster reef
has become the most endangered marine ecosystem in the world. Oysters also suffered from
the introduction of a parasite from the western Pacific after the Korean War. This causative
agent of MSX disease caused 90% mortality in oyster populations in the lower Delaware and
Chesapeake Bays. The clearing of forests for agriculture throughout east coast watersheds
resulted in soil erosion and subsequent sedimentation of estuaries smothering shellfish and
other benthic (bottom) fauna. Brown tides of the past few decades in New York and New
England brought sharp declines in clam and oyster populations and almost eliminated bay
scallops in some localities. Introduced predators of shellfish such as the Rapa whelk and the
Chinese mitten crab and a number of fouling species of sea squirts are more recent threats to
shellfish. Ironically the first English settlements in the lower Chesapeake estuary probably would
not have survived the first few years had it not been for the abundant nearby shellfish.
But perhaps the most serious threat to shellfish is just beginning to manifest itself – ocean
acidification. By this time most people are aware that the burning of fossil fuels and the resulting
release of carbon dioxide, CO2, into the air is causing major changes in the world’s climate. The
most notable aspect of this is a general warming of the earth’s surface. A significant portion,
over 25%, of the CO2 that has been released has been absorbed by the oceans. CO2 combines
with H2O to form H2CO3, carbonic acid, and this is causing a lowering of the oceans’ pH, i.e. it is
becoming more acidic.
The pH scale is a way of measuring the acidity and alkalinity of a solution. The scale runs from
0 to 14. The middle of the scale is 7, considered neutral (neither acidic nor alkaline.) As the
solution goes up the scale toward 14 it becomes more and more alkaline. As the solution goes
down it becomes more and more acidic. It is a logarithmic scale so an increase or decrease of a
whole integer means the pH increases or decreases by a power of ten. Thus a pH of 4 is ten
times more acidic than a pH of five. A pH of 11 is 100 times more alkaline than a pH of 9.
Acidification is a relative term. Even though the oceans remain alkaline, the pH is lower than it
used to be and therefore more acidic. (A temperature of -10° is very cold but it’s still warmer
than -20°.)
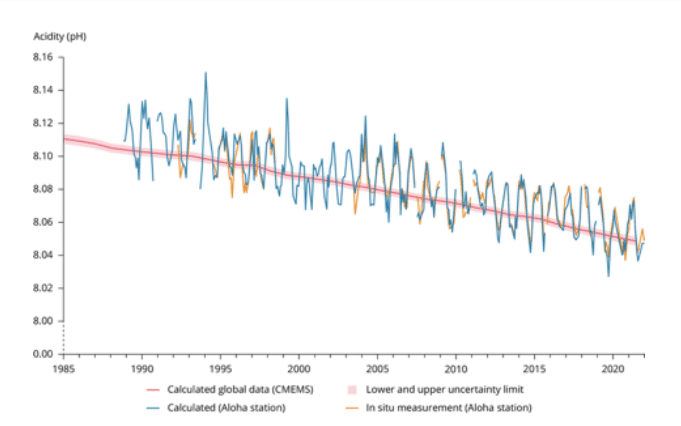
The oceans’ pH has already fallen from 8.25 to 8.14 (a drop of 30%) and may reach 7.85 by the
end of this century, more acidic than the oceans have been in the last 20,000,000 years.
So, to see how this is affecting shellfish let’s have a quick look at how mollusks form their shells.
There’s a lot that is not known about shell deposition but this is a general summary. Outside the
body of the mollusk, between the body and the edge of the shell (extrapallial space) the shellfish
maintains calcium carbonate (CaCO3) in a very high concentration. This calcium carbonate
comes from seawater. With the addition of nitrogen waste from the organism in the form of
ammonia, the pH is raised to the point where the calcium carbonate begins to form crystalline
granules and drop out of solution. These continue to grow and crystallize into large calcite
crystals which are then embedded in a protein matrix that exudes from special glands and
hardens in water. These CaCO3 crystals in a protein matrix are then added to the shell.1
1 During the colder months very little shell deposition occurs so that annual growth rings are
usually visible like tree rings.
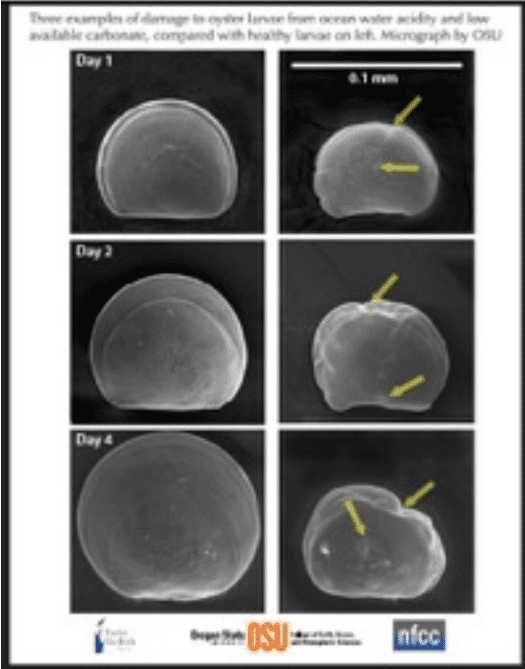
As the pH goes down, i.e. as acidification increases, the shellfish is threatened in two ways.
(1)The shell itself will begin to dissolve and become thinner and (2) it becomes more difficult
(more energy must be expended) for the organism to form the shell. Recent research suggests
that estuarine shellfish are more susceptible that ocean shellfish in that estuaries are already
more acidic than oceans. Many species of young shellfish are affected more than mature
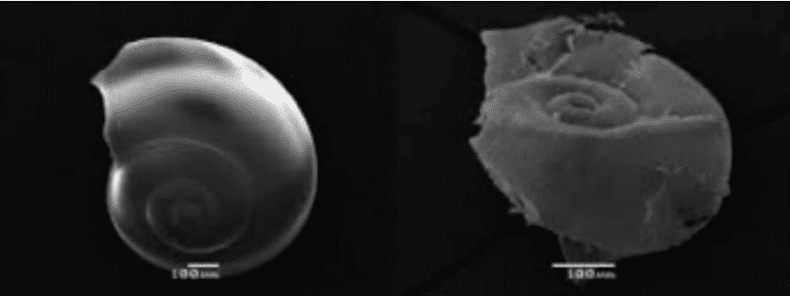
shellfish. For example, young larval oysters in the veliger stage rely on a crystalline form of
CaCO3 (aragonite)that is more soluble at a lower pH than the crystalline form found in mature
oysters (calcite). But even adult oysters will show retarded shell growth.
Many species of marine organisms other than shellfish rely on CaCO3 deposition to survive.
Certain one-celled planktonic organisms, corals, planktonic molluscs and sea urchins are but a
few. Some fish larvae succumb to water of a pH expected by the end of this century. Even the
behavior of adult fish may be altered.
Blog #3 – The Emerald Elysia
The eastern emerald elysia, Elysia chlorotica, has my vote as the most interesting organism of the wetlands bordering the Delaware Bay. This sea slug is broad, flat, bright green and photosynthesizes like a leaf but is more closely related to a snail than to any plant.
Before I go any further let me digress and explain a little about photosynthesis. Photosynthesis is how green plants and algae and some microorganisms make their own food. Taking water (H2O) and carbon dioxide (CO2) from their environment and using light energy from the sun, plants are able to manufacture carbohydrate (C6H12O6) which they then use for energy to accomplish many metabolic functions. Animals, unable to make their own carbohydrates, eat plants or other animals and convert their prey’s carbohydrates to energy. This conversion of CO2 and H2O into carbohydrate is not done just anywhere in the plant but is confined to cellular structures called chloroplasts where the green pigments that do much of the work of photosynthesis are found.
1“Sea Slug is not a taxonomic term but is used to describe a number of groups of Mollusca including nudibranchs, sap-sucking sea slugs, bubble shells, sea hares and sea angels.
Way, way back in evolutionary history, about a billion years ago and about the time that plants diverged from animals, a cell with a nucleus ingested a photosynthesizing bacterium. Instead of digesting this bacteria and using its chemical constituents as nutrients, the bacteria remained viable and continued to produce carbohydrates. These bacteria were the first chloroplasts. The chloroplasts of green plants today are more bacteria-like than plant-like in their DNA and protein assembly structures, and are capable of reproducing independent of the plant cell’s replication. Over time the nucleated cell acquired the DNA that was then translated into proteins that supported the chloroplast. So here we have a symbiotic relationship, one in which both the plant and the bacteria-like chloroplast have benefited. O.K. Back to Elysia. The eastern emerald elysia lives in shallow pools on salt marshes. Its size is from an inch to several inches in length and it feeds on the long filaments (the cells are tube-like and connect end to end) of the alga Vaucheria litorea (sorry, it has no common name that I know of). The elysia bites off the end and sucks the contents out. Most of the alga’s contents are digested but the chloroplasts are sequestered in special cells of the highly branched digestive tract of the sea slug. Here they continue to photosynthesize for as much as a year. As a juvenile, elysia is a yellowish brown but turns a bright green as it accumulates chloroplasts. Adults can live months and through the winter on the food these chloroplasts produce.
AND this is only half the story! Photosynthesis supporting genes in Vaucheria’s chromosomes are also stolen by the elysia, incorporated into the sea slug’s chromosomes, and used to continue and maintain its carbohydrate production. This horizontal gene transfer, as opposed to vertical gene transfer from parents to their offspring is fairly common in bacteria but it is rarer in higher organisms though not unknown. Elysia is an hermaphrodite, meaning individual adults are both male and female. (Much more about hermaphrodites in later blogs.) In the spring, after the adult has laid its egg masses, all adults die of a virus that the individual carries with it throughout its life. This synchronous death even occurs in laboratory reared specimens. Elysia is uncommon and seems to be getting rarer but it is possible to spot them in the shallow pools in which Vaucheria lives. Both Elysia and Vaucheria can tolerate a broad range of salinities from seawater to brackish, almost freshwater.
Blog #2 – The Common Sea Star
One of the oddest feeding mechanisms in nature can be observed in the common sea star, Asterias forbesi, the most numerous of the Delaware Bay sea stars. To ingest its food the sea star everts its stomach (turns its stomach inside out) through its mouth, grabs its prey with the muscular stomach, and pulls the stomach with its meal back into its mouth. If this is a little hard to imagine, here’s a Youtube link where you can watch the process. https://www.youtube.com/watch?v=l6dnmLDu6Eg
It feeds on snails, barnacles and other small crustaceans, and bivalves (dead or alive) such as clams, oysters and mussels. Feeding on a live bivalve is a bit of an art for the starfish. One of the persistent explanations of how a starfish opens a bivalve is that a starfish releases an anesthetic that relaxes the adductor mussels causing the valves (shells) to gape open.
Asterias forbesi, the common sea star.Kennedy, Newell and Eble, in their 1996 book on the Eastern oyster mention this anesthetic but I have not been able to find the actual chemical structure or, indeed, if anyone has even isolated such a chemical. My search has admittedly been limited but I’m beginning to doubt the anesthetic’s existence. If any readers know that such an anesthetic has been isolated, please let me know.
More likely, the starfish is able to separate the valves by latching on to the valves with its suction-cupped tube feet on the bottom of its arms and pulling. When the valves open a tiny fraction of an inch, 0.1 mm, about the width of a period on this page, the starfish can then evert its stomach which squeezes into the gap and releases digestive enzymes. Even if the starfish cannot maintain this pulling pressure for long and the bivalve slams shut, the starfish will keep pulling until, little by little, the stomach is able to expand into the bivalve. Then digestion proceeds to the point that the bivalve relaxes and gapes open.
The common sea star’s predation on bivalves can be extensive. It is an important predator in the Chesapeake and Delaware Bays and even more important predator north of the Delaware Bay. Predation on oyster spat (young oysters) by the common sea star has sometimes exceeded 90% in Long Island Sound. In the days when many sailing vessels, Sharpies and Schooners, were dredging oysters from the Sound, the common sea star was so important a predator that oystermen would drag sea mops over the floor of the Sound. These mops consisted of hundreds of lengths of rope attached to a 10’ – 20’ long bar of steel. As these mops were dragged through the bottom of the sound, the sea stars would attach to or become entangled with the ropes. The crew would haul the mop to the deck, chop the sea stars in pieces and throw the remains overboard.
This, of course, backfired! Starfish are capable of regeneration and only 20% of a starfish is needed to grow into a whole new starfish. It reminds me so much of Mickey Mouse as “The Sorcerer’s Apprentice” in Disney’s film “Fantasia”. Mickey couldn’t stop the broom from carrying buckets of water so he chopped the broom into splinters and each splinter carried the buckets. The oystermen and clammers soon recognized the errors of their ways and rather than returning the pieces of starfish to the Sound, they sold them instead as a chicken feed supplement. Whether or not these efforts resulted in a reduction in predation and an increase in shellfish numbers has never been determined.
Strangely (to me) starfish and other Echinoderms (the phylum to which starfish belong) are more closely related to Chordates and therefore to us than they are to other invertebrates. Their biology, including their life cycle and unique water vascular system I hope to include in another posting. By the way, that little red disc, seen just off-center in the first picture, is an organ used to take water into the sea star’s water vascular system. (Sea Star’s have no blood.)
Blog #1 – Mile 0
My favorite stretch of beach in New Jersey is Cape May Point. It’s a great place to see dolphins and ghost crabs, whelk egg cases and sanderlings, and the thousands of other natural curiosities that draw me back there at any season. When there, I never fail to see someone in a T-shirt that reads “ Mile 0”, a reference to the beginning of the NJ Garden State Parkway.
But there’s another important Mile 0. If you were to draw a line from Cape May Point, NJ to Cape Henlopen, Delaware, you would define the boundary between the Delaware Bay and the Atlantic Ocean. From Mile 0 it is 330 miles north to the confluence of the East and West Branches of the Delaware River at Hancock, NY. The Delaware is the only major un-dammed river in the Eastern U.S. and it drains an area of over 13,500 square miles in five states (though only a few square miles of Maryland) . A river mileage system has been in use for the past fifty years to describe locations upstream of Mile 0.
Besides mile 0 there are two other important lines. One is visible and immovable, the other invisible and moveable. The immoveable one is called the Atlantic Seaboard Fall Line and occurs at the falls of the Delaware, Mile 133, just below the “Trenton Makes the World Takes” bridge. In a relatively short span the river drops about eight feet in elevation and separates the unidirectional flow of the river from the tidal portion of the river. The second, moveable line is the Salt Front. The Salt Front occurs when the concentration of the chloride ion reaches 250 milligrams per liter. This is based on drinking water quality standards. At times of low freshwater input, such as in periods of little rainfall, the Salt Front moves up the Delaware. Nineteen sixty-four was a drought year and by November of ’64 the Salt Front had moved up to River Mile 102, just north of the Benjamin Franklin Bridge. At the time of this writing in January, 2024 the Salt Front was at River Mile 54, south of where the C & D Canal enters the Delaware. Both the Fall Line and the Salt Line will impact the kinds and distributions of many organisms that live in these waters.
In the coming months we’ll be looking at the many creatures of the Delaware – who they are, where and how they live and how they interact with one another. My only purpose in writing these blogs is to share the joy and fascination that I’ve found exploring the ecology of the Delaware Bay and River.
I would greatly appreciate corrections, comments and questions.
Patrick “Pete” Slavin has lived within the Delaware River watershed most of his life. He holds a PhD in Ecology from Rutgers and has worked in public health, agriculture and academia.